FDA injunctions offer window into problems facing dietary supplement industry
Most supplement firms that were sued or threatened with a complaint agreed to cease operations under agreements that were reached with the Justice Department.

This is the first in a series of articles on civil and criminal enforcement actions that have been taken against dietary supplement firms in recent years.
Sometimes, government officials overseeing America’s dietary supplement industry move to permanently shut down a company that has violated federal regulations. Since the fall of 2010, FDA and the U.S. Justice Department have secured permanent injunctions against at least 15 companies that made and marketed dietary supplements.
Most supplement companies that were sued or threatened with a complaint agreed to cease operations under agreements that were reached with the Justice Department. The few companies that mounted a defense eventually capitulated to the government or failed to sway the federal judge overseeing the case.
The civil lawsuits offer a perspective into the types of corporate malfeasance in the supplement industry that FDA considers the most egregious and likely a legitimate threat to public health. In several complaints seeking an injunction, the government accused supplement marketers of making claims that their products treat diseases such as autism, Alzheimer’s, epilepsy and skin cancer. Public health officials fear consumers who take such mislabeled products will forgo or delay proper medical treatment.
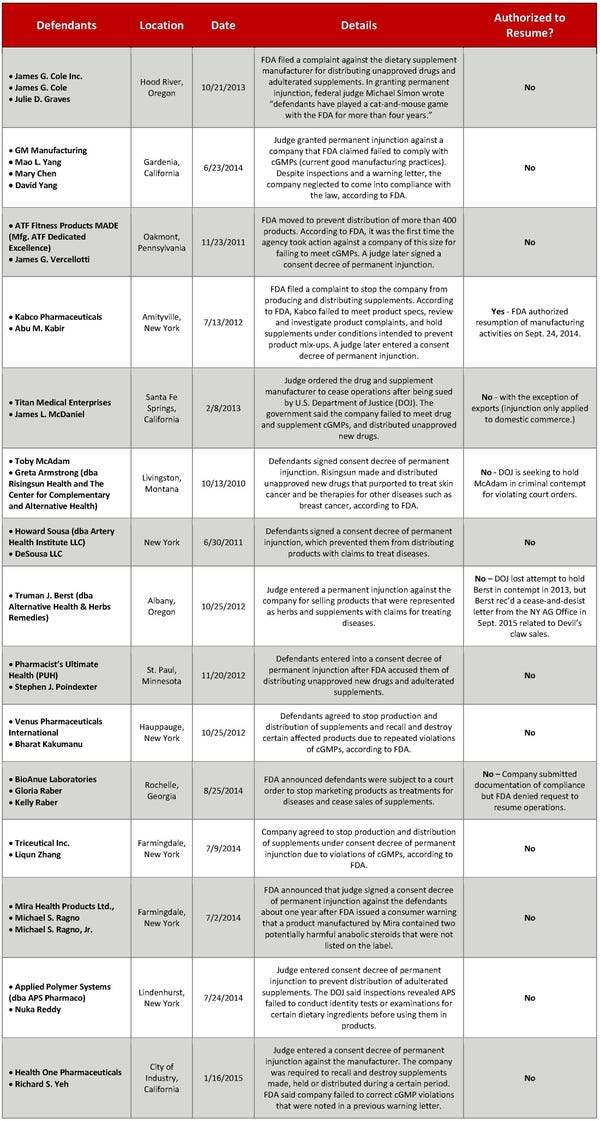
Some of the same supplement companies that made alleged drug claims—and others—were accused of repeatedly violating manufacturing regulations that FDA adopted eight years ago. Another company was shut down after FDA announced that a lab analysis revealed a product contained potentially harmful anabolic steroids.
FDA also has used the judiciary to seize potentially dangerous supplements. In recent years, federal authorities have seized supplements containing DMAA from two large companies, including the national retailer GNC. According to FDA, the substance has been linked to more than 100 illnesses, including several deaths. DMAA also is the subject of ongoing litigation in private and government proceedings.
Over nearly the last five years, additional seizures have targeted probiotic products that were marketed as drugs, raw materials containing banned ephedrine alkaloids, and other products that were marketed to treat diseases including asthma, cataracts, gangrene and osteoarthritis.
Last year, FDA seized roughly 25,000 pounds of raw kratom, a botanical substance that grows naturally in Southeast Asia. The government said the botanical substance is used for the treatment of heroin and morphine addiction but has no acceptable medical uses.
The civil lawsuits exclude administrative actions that were taken, such as when FDA temporarily detained supplements containing DMAA before the federal courts authorized the U.S. Marshals Service to seize the products.
Adequate enforcement?
Attorneys general and federal lawmakers have questioned in recent months whether FDA exerts adequate oversight over the supplement industry and whether the government is sufficiently aggressive in its enforcement. Several industry representatives insisted that the civil enforcement actions demonstrate the government has ample legal authority to crack down on wrongdoers.
The more important question, former FDA official Daniel Fabricant, Ph.D., said, is the extent to which transgressors are effectively, swiftly and thoroughly “brought to some form of justice."
That question was recently raised after researchers led by the physician Pieter Cohen, M.D., of Harvard Medical School found that an amphetamine-like substance known as BMPEA was being marketed as a dietary supplement well after FDA had studied the issue. In the face of pressure from lawmakers, FDA issued warning letters to a number of companies marketing the substance.
Responding at the time to criticism by Cohen that FDA hadn’t acted quickly enough, an agency spokesperson, Siobhan DeLancey, said, “The FDA prioritizes enforcement actions based on available resources and the level of safety concern identified; the agency faces the challenge of having limited resources to monitor the marketplace for potentially harmful dietary supplements."
Fabricant led FDA’s Division of Dietary Supplement Programs from February 2011 until April 2014. He said his division during his tenure brought more injunction actions than any other division in the agency.
“Injunctions, while they are important, are incredibly intensive from a research perspective," said Fabricant, who presently serves as executive director and CEO of the Natural Products Association (NPA). “I think seizures have a better deterrent effect. I also think in some instances some of these cases could have been elevated sooner."
Of all the enforcement tools at FDA’s disposal, the agency relies most often on warning letters. Over nearly a five-year period, FDA issued approximately 300 warning letters to supplement companies, according to sources that track the letters.
Tainted supplements
Steve Mister, president and CEO of the Council for Responsible Nutrition (CRN), raised concerns that FDA is not being aggressive enough in its enforcement actions against products that are tainted with pharmaceutical ingredients and other potentially dangerous substances. Mister referenced dozens of warning letters in which products marketed as dietary supplements were said to contain prescription drugs or drug analogs.
“It is hard to conceive somebody did not put that in there intentionally," said Mister, who wants FDA to elevate its enforcement action against such products beyond warning letters. “Somebody in that supply chain knew what they were doing. Viagra does not end up in an herbal product by accident."
“Where are the formal recalls and seizures of those products?" Mister asked in a phone interview. “It would seem like to me the agency should move quicker to recall those products, seize them, destroy them so they cannot get into consumers’ hands."
Over the last decade, FDA has found more than 500 over-the-counter (OTC) products that contain potentially harmful ingredients, an FDA spokesman, Stephen King, said. The tainted ingredients are often found in products that are marketed as supplements for bodybuilding, sexual enhancement and weight loss.
“FDA has been alerting the public of these deceptive products via ‘Immediate Public Notifications’ for approximately four years and through email outreach," King said in an email.
Such products are misbranded drugs, not supplements, pointed out Michael McGuffin, president of the American Herbal Products Association (AHPA).
“I don’t know who those scoundrels are," McGuffin said in a phone interview. “They don’t join trade associations."
“We encourage the strongest and most aggressive enforcement by the agency and by the Department of Justice the first time," McGuffin added. “We don’t want to see hand slaps. We don’t want to see warning letters. We want to see criminal convictions, and we’ve seen a few of those."
Earlier this spring, Sens. Orrin Hatch (R-Utah) and Martin Heinrich (D-New Mexico) urged the Justice Department to take criminal action against individuals and companies that sell supplements that are tainted with anabolic steroids, active pharmaceutical ingredients or related analogs. Warning letters are not sufficient to deter criminal behavior, Hatch and Heinrich wrote in a letter to U.S. Attorney General Loretta Lynch.
“We applaud the positive results that have come on the occasions that DOJ and FDA have jointly pursued criminal cases of this nature, especially those that have led to successful convictions," the senators wrote, referencing a recent case in which a 53-year-old man was sentenced to nine years behind bars for selling erectile dysfunction drugs that were marketed as supplements. “We note, however, that the low number of such criminal convictions has had a limited impact on deterring would-be criminals."
Vasilios (“Bill") Frankos, Ph.D., a former supplement director at FDA who presently works at Herbalife Ltd., said when he was at the agency it was challenging to take enforcement action against manufacturers of tainted supplements because products were often manufactured overseas in far-flung places like Southeast Asia and sold over the Internet.
“It was hard back then to find these people," he said.
As of deadline, an FDA spokesman did not respond to multiple requests for comment on criticisms that the government should be more aggressive in its enforcement action against marketers of tainted products.
Manufacturing violations
Tainted or spiked products are not the only reason critics have viewed the supplement industry with skepticism. Supplement firms are continuing to struggle to meet cGMPs (current good manufacturing practices), even though the regulations have been in effect for even the smallest companies for the last five years. As Natural Products Insider previously reported, FDA data showed six in 10 dietary supplement facilities that were inspected by FDA in FY14 failed to fully comply with cGMPs. Nearly one in five facilities that was cited by FDA for noncompliance with regulations following an inspection purportedly failed to verify the identity of a dietary ingredient prior to use through a test or examination; the identification omission reflected the most common infraction cited by FDA inspectors, the data showed.
FDA has successfully shut down at least 11 companies that haven’t followed the cGMPs. Still, Fabricant said government lawyers are more reticent to bring an enforcement action in connection with cGMPs (versus a case of microbacterial contamination) due to the technical nature of the manufacturing requirements.
While FDA has brought a relatively small number of injunction proceedings against supplement firms for violating cGMPs, the agency hasn’t been stingy on ink. Since 2009, FDA has issued around 325 warning letters to supplement firms in connection with cGMPs, Fabricant noted. He agreed FDA should take prompt action if an inspection reveals that a company is woefully out of compliance with the manufacturing regulations.
“If that degree of compliance is nonexistent," Fabricant said, “there should be a swift movement to get that firm reinspected and initiate further action."
Mister said products that are not made in compliance with cGMPs are “per se adulterated" under the Federal Food, Drug & Cosmetic Act (FD&C). He said that is an important distinction from other foods because the government is not required to show the supplement is adulterated in some other manner.
“It should be very easy for FDA to bring adulteration violations against a dietary supplement company because they don’t have to show it causes harm in the marketplace," he said. “All they have to show is, ‘these are the rules and you didn’t follow them.’"
Collaboration with DOJ
Still, FDA supplement officials who want to take enforcement action cannot act unilaterally. They must secure the approval of several officials within the agency, as well as at the Justice Department.
Fabricant said a potential enforcement action would first be reviewed by numerous FDA interests, including the compliance and investigation branches. Any enforcement action that was taken at the center overseeing supplements also would need to be reviewed by the Office of the Chief Counsel, Herbalife’s Frankos noted. The Office of the Chief Counsel acts as liaison to the Justice Department during active litigation, and often will serve as special assistant U.S. attorneys, he explained.
“Very little could get done," Frankos said, “unless you got the full support of the Office of Chief Counsel."
He said the Justice Department prioritizes its resources in the supplement realm based on whether there is a danger to public health.
“Unlawful activity in the dietary supplement market is a focus of the Justice Department’s consumer protection efforts," said Nicole Navas, a Justice Department spokeswoman, in an email. “As can be seen in recent enforcement actions, the department’s Consumer Protection Branch is working closely with the FDA and FTC in this area."
Undue delays?
Earlier this month, the Justice Department filed a civil complaint against Iowa Select Herbs, a supplement distributor and manufacturer. According to the government, the company has made unlawful disease claims and repeatedly neglected to test its dietary ingredients before using them.
The case is emblematic of the years of back-and-forth discussions between FDA and a supplement company that is allegedly out of compliance with the law.
Following an August 2013 inspection, FDA first notified Iowa Select Herbs that it was out of compliance with cGMPs and warned the company’s president, Gordon Freeman, that failure to comply with the law could result in an injunction, according to the complaint. Eight months later, based on the inspection and a review of the company’s website, FDA issued a warning letter to Iowa Select Herbs.
Freeman responded to FDA, promising that the company “had made many corrections," but a subsequent inspection last summer revealed “significant violations," according to the lawsuit. Although FDA had issued a Form 483 that cited 13 observations or alleged violations of FDA regulations, the Justice Department claimed the company still had not responded to the agency as of the date of the complaint.
Freeman, whose Cedar Rapids, Iowa business is nearly 25 years old, told Natural Products Insider that the FDA investigator never told him that he needed to send the agency evidence of compliance with the regulations. Although Iowa Select Herbs has retained an attorney, Marc Sanchez, Freeman said he didn’t plan to contest the government’s action.
“The cost involved has pretty much put me out business," said Freeman, who noted that he is a disabled Army veteran who served in Iraq. “I hate to say it but you can’t fight Big Brother. If you do, you are expecting to pay out a large amount of money. Every single month, we barely make ends meet."
In an email, Sanchez said, “At this time, we are gathering information and working with the DOJ to find an amicable solution. There should be a further update on the matter either from myself or the DOJ in the next week or two."
In a July 19 editorial, The Des Moines Register cited the case against Iowa Select Herbs as “a strong reminder of just how little oversight the government has when it comes to the $33 billion supplements industry."
In a letter submitted this week to the newspaper, supplement lawyer Marc Ullman responded to criticism that the 1994 Dietary Supplement Health and Education Act (DSHEA) is a weak law that protects industry and hampers government.
“Because the products sold by this company make illegal ‘drug claims,’ as your editorial recognizes, they are considered illegal, unapproved new drugs thus removed from anything related to DSHEA," Ullman of the New York law firm Ullman, Shapiro & Ullman LLP wrote in the letter.
Ullman also addressed criticism that FDA is too slow to take enforcement action and recent newspaper editorials that have questioned the prudence of former industry representatives moving over to FDA to oversee its supplement division.
Fabricant and Cara Welch, Ph.D., were both NPA executives before they joined FDA’s Division of Dietary Supplement Programs. Bob Durkin is FDA’s current acting supplement director. As a long-time FDA employee who is trained as a pharmacist and lawyer, Durkin has never worked for a dietary supplement trade association.
“The blame for the [eight] months that elapsed before FDA finally issued a Warning Letter to this company is the result of the maze of byzantine procedures that regulators like Drs. Fabricant and [Cara] Welch are forced to navigate before they are able to move FDA’s lawyers to approve any agency enforcement action," Ullman wrote. “Similarly, the passage of an additional year before commencing an injunction action can be traced to the need for the same FDA lawyers to convince local prosecutors to file such an action."
About the Author
You May Also Like

