Kabco Pharmaceuticals: Story of redemption in dietary supplement sector
Of 15 dietary supplement firms whose operations have been shuttered by FDA over the past five years, the New York-based company is the only manufacturer of natural products that has been given permission to return to the business.

This is the fourth blog in a series of articles on civil and criminal enforcement actions that have been taken against dietary supplement firms in recent years.
Kabco Pharmaceuticals Inc., a contract manufacturer of dietary supplements, had to shut its doors in 2013 for violating FDA regulations.
Many companies facing a similar predicament would have called it quits permanently. Kabco may be an anomaly.
Of 15 dietary supplement firms whose operations have been shuttered by FDA over the past five years, the New York-based company is the only manufacturer of natural products that has been given permission to return to the business, according to records Natural Products INSIDER obtained this summer through a Freedom of Information Act request.
“I think there is a message here, which is the injunctions are appearing to have an effect in the marketplace," said Steve Mister, president and CEO of the Council for Responsible Nutrition, in a Sept. 30 phone interview. “When FDA pursues these things through beyond the warning letter, they can have an effect and deterrent in the industry."
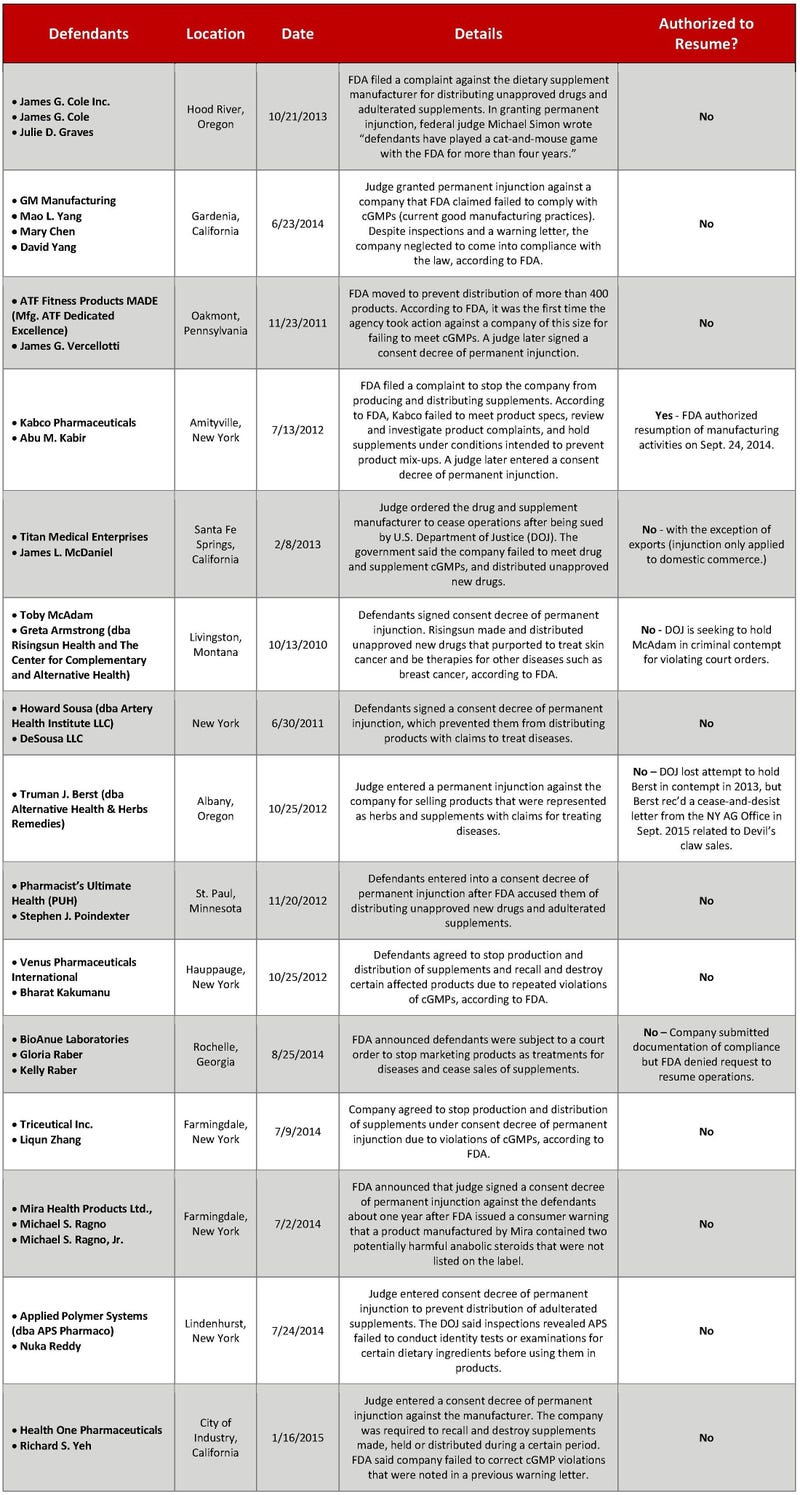
Kabco: A ‘completely different’ operation
On Sept. 24, 2014, FDA authorized Kabco to resume operations, though the company remains subject to the terms of a court order or so-called consent decree, according to FDA’s New York District. Rezaur R. Yousuf, Kabco’s chief operating officer, described his company in a phone interview as one that has transformed itself in an effort to meet FDA regulations, produce quality products and rebuild customers’ trust in its manufacturing operations.
“For us, what has happened in the past is gone," said Yousuf, who recently explained the company was audited last month by NSF International and is close to obtaining GMP (good manufacturing practice)-registration through the third-party auditor. “The whole operation is completely different."
Obtaining permission from the government to resume operations heralded a new round of challenges: hiring the right people, training them and winning back customers.
“More and more customers," Yousuf said, “are beginning to regain confidence in us."
Other dietary supplement firms that have been sued by the U.S. Department of Justice (DOJ) have not been so fortunate. A number of them have gone out of business permanently. Others have purportedly defied court orders and started operations again, illustrating myriad challenges FDA faces.
Dietary supplement firms that are subject to an injunction typically have a chance to revive their businesses, subject to the following caveats: They must implement a number of measures that are intended to ensure compliance with FDA regulations, and obtain the agency’s blessing to resume operations. A number of companies may reckon the costs and burdens of meeting the government’s terms aren’t worth the effort.
“If you were starting from zero compliance … that’s an enormous job to take a company like that and bring it into compliance," said Anthony Young, a veteran food and drug lawyer with Washington-based Kleinfeld, Kaplan & Becker LLP, in a phone interview. (Young commented on Kabco’s closure years ago).
“For a company that makes a lot of products, it’s going to be a tough job" and “very expensive," added Young, who noted the daily cost of hiring a consultant alone can total several hundred dollars. “I am not surprised that a lot of companies just simply give it up and go into another line of work."
Three New York-based dietary supplement manufacturers are a case in point. The businesses were unable to come into compliance with consent decrees and have permanently ceased operations, according to attorney Marc Ullman, who represented the firms and recently joined Rivkin Radler LLP as Of Counsel.
Asked why companies throw in the towel, Kabco’s Yousuf commented, “The companies have been caught. They figured out they have made enough money. They do not want to spend" more funds to come into compliance.
“It’s all about the mindset," he said. “If you want to do well and the intent is there … you can do no wrong. The FDA is not your enemy."
Supplement marketer foiled in effort to resume operations
BioAnue Labs may disagree. In spite of writing to lawmakers and FDA, the marketer of natural products failed to persuade regulators that it has complied with the terms of a consent decree. BioAnue in Rochelle, Georgia was shut down last summer after FDA accused the company of marketing supplements as drugs and manufacturing products out of compliance with cGMPs.
Within two weeks of the injunction being entered, the company obtained verification from a consultant that it was in compliance with FDA regulations, according to Kelly Raber, who was named as a defendant in the injunction case along with his wife Gloria and BioAnue. Although the information was communicated to the government, FDA wasn’t persuaded.
“We asked for clarification, and they said, ‘We owe you none,’" Kelly Raber said in a phone interview this summer. “That’s where we’re standing today. So basically, what I’d call them is bullies."
DOJ trial attorney Charles Biro advised BioAnue’s counsel, John Harrington, in a Dec. 19, 2014 letter that the company had not established compliance with requirements set forth in the court order.
Although defendants’ websites had been modified, they “still contain content claims that cause their products to be drugs," and BioAnue’s expert failed to certify the defendants removed all claims that caused the products to be drugs, Biro advised Harrington. He also said the expert’s virtual or remote GMP inspection was inadequate.
Ralph Fucetola, a consultant in New Jersey that BioAnue retained who practiced law for 35 years and was known according to his website as “The Vitamin Lawyer," conducted a virtual audit of BioAnue’s facilities via Skype, according to Biro’s letter.
“Defendants’ cGMP expert must be able to freely inspect the BioAnue facility with full control over what he is or is not seeing," Biro wrote. “The remote audit that Mr. Fucetola describes does not offer him the firsthand sensory experience necessary to adequately and comprehensively inspect the physical conditions at the BioAnue facility."
In a phone interview, Fucetola said he uses a checklist of 195 items during his virtual audit. Other than the sense of smell, Fucetola perceives no difference between an onsite audit and virtual one. FDA’s distinction is one “without meaning that results in small companies being forced out of the market," said Fucetola, who explained an onsite audit is significantly more expensive than a virtual one because it requires travel.
BioAnue wrote to members of Congress and even sought clarification on what it needed to do under its consent decree to resume operations. Its outreach prompted the DOJ to write to Sen. Johnny Isakson (R-Georgia) earlier this year. Acting Assistant Attorney General Joyce Branda referenced Biro’s letter which “discusses the very clarifications that Ms. Raber asked you to provide."
Kelly Raber said he and his wife aren’t giving up, though he added, “I’m just not going to waste my time trying to contact Congressmen every 10 days."
FDA: Some firms ignoring injunctions
Other dietary supplement marketers that have been subject to an injunction in recent years have returned to federal court for allegedly doing the very thing the court order prohibited: selling natural products.
According to FDA’s Seattle District, Alternative Health & Herbs Remedies (now known as Evangelical Christian Ministries) has not been authorized to resume operations. But as INSIDER reported last month, Albany, Oregon-based Health & Herbs—a self-described church outreach of Evangelical Christian Ministries—is marketing supplements after the DOJ failed to persuade a federal judge to hold in contempt Truman Berst, the owner of Alternative Health & Herbs Remedies. Berst, who recently found himself in the crosshairs of New York Attorney General Eric Schneiderman, advised a judge in 2013 that the herbal business had been sold to his daughter.
Elsewhere in the Northwest, a businessman who sold dietary supplements is facing the unwelcome prospect of incarceration. The DOJ is seeking to hold Toby McAdam of Livingston, Montana-based Risingsun Herbal Health Corp. (also doing business as Risingsun Nutritional Corp.) in criminal contempt for selling dietary supplements and drugs in violation of court orders.
In fall 2010, FDA announced that McAdam and another person had “signed a consent decree that prohibits them from manufacturing and selling unapproved new drugs and adulterated or misbranded dietary supplements in violation of the law." The government later sought to hold McAdam in civil contempt, a request that was granted on Dec. 4, 2013 by a federal judge and later affirmed by an appeals court.
Last week, U.S. District Judge Susan Watters granted the government’s petition to show cause why McAdam should not be held in criminal contempt. According to the government’s request to hold McAdam in criminal contempt, he has failed to close Internet businesses on Amazon.com, websites and a promotional Facebook page he uses to promote his products.
The DOJ filed a charge against McAdam that carries a penalty of up to six months in prison. The government alleged in court papers that McAdam sold a dietary supplement (Bloodroot Immune Support Capsules) to an undercover FDA agent on April 23, 2015; he also sold Lugol’s Iodine to an undercover employee through Risingsun’s Amazon.com storefront, according to the court documents.
McAdam has insisted he’s done nothing wrong. In Aug. 4 court papers filed in Montana, McAdam argued he’s selling “Lugol’s iodine," not a dietary supplement. McAdam also contended the government’s case against him is “moot" and should be “stayed" before the U.S. Supreme Court addresses issues he raised in a petition filed with America’s highest court. (The Supreme Court reportedly accepts less than 1 percent of petitions for certiorari asking it to review a case).
In rejecting McAdam’s arguments, the DOJ noted two courts—including the U.S. Court of Appeals for the Ninth Circuit—previously denied his requests for a stay of the civil contempt order. The government also insisted the iodine product is a dietary supplement because it is “intended to supplement the diet that contains a mineral and is intended to be ingested as a liquid."
The criminal contempt case against McAdam remains pending five years after the injunction against him was entered.
About the Author
You May Also Like

