Overview of the European Supplement and Novel Foods Market
The European food supplement market is projected to grow by around 9.5 percent in the next few years, but entering the market requires product registration and an understanding of regulatory pathways.
May 31, 2016

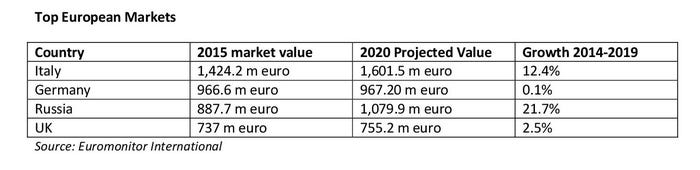
According to data from Euromonitor International, the European food supplement market is projected to grow by around 9.5 percent in the next few years—estimated to be a €7.9 billion market value by 2020. The top three European countries in food supplement markets are Italy, Germany and Russia. However, Eastern Europe markets are grown rapidly. Belarus’s food supplement market increased by almost 400 percent between 2010 to 2015, from just €3.1 million to €15.5 million in 2015. According to Euromonitor’s forecast data, Eastern European countries such as Romania, Turkey, Bosnia-Herzegovina, Russia and Macedonia are projected to be the top fastest-growing markets.
According to Euromonitor data, the top 10 European markets for food supplement are Italy, Germany, Russia, UK, France, Poland, Norway, Finland, Belgium and Spain.
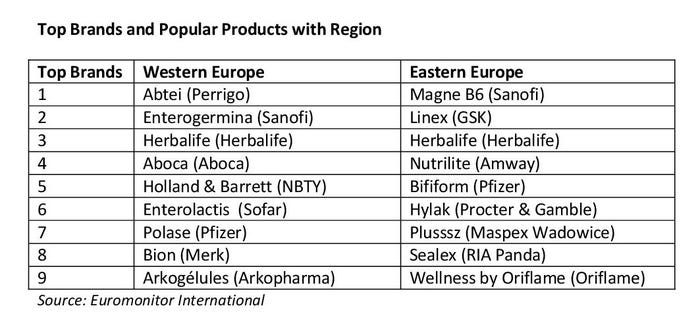
Popular products for Western Europe are related to digestive health, general well-being, bone health, sexual health, immunity, heart health and beauty.
Regulation
In the EU generally, there are two types of product registration: food supplements and novel foods.
• Food Supplements
Food supplements are regulated in the EU by the Directive 2002/46/EC of the European Parliament of 10 June 2002, which provides the definition of food supplements as “foodstuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities."
• Novel Foods
The novel foods category is an emerging field, and companies may wish to introduce novel foods because they are produced more efficiently and/or because they might have advantages for the health and nutrition of consumers.
Novel food is regulated by the Novel Foods Regulation (Regulation (EC) No 258/97), and is defined as a “food that does not have a significant history of consumption within the EU before 15 May 1997." Acquiring a novel food classification might be a lengthy process, however if this route is chosen, it is important to remember that the Novel Foods Regulation includes a simplified procedure for marketing certain types of novel food or novel food ingredients in the EU if it is considered “substantially equivalent" to an existing food or food ingredient that is already marketed within the EU. This procedure is still in place, however, there are plans to review it. Typically, composition (such as the source organism and preparation method), nutritional value, metabolism, intended use (such as a food ingredient or supplement), level of undesirable substances (such as contaminants, mycotoxins and allergens) would be investigated. In these cases, the company can submit a notification to the European Commission after obtaining an opinion on equivalence from an EU Member State.
Market Strategy
Typically start the notification process in Belgium because Belgian law is the most advanced in terms of substances that can be present in food supplements, notably botanicals. Generally, it takes six to nine months to gain a product registration. Once a product is authorized in one EU country, then the mutual recognition principle is applied when notifying in another EU country. However, sometimes, this principle does not work properly, as other EU countries consider that the ingredient presents a risk to human health, it’s medicinal, etc. Local laws would apply in that case.
This information is for reference only. It has no legal value. Davidia Healthtech LLC declines all responsibility or liability for errors or deficiencies in this document. Authorities in various countries have the right to determine a product’s regulatory status. Laws and the related guidance change constantly. We cannot guarantee regulations referred to in the document remain unchanged. The text should not be taken as an authoritative statement or interpretation of the law, as only the courts have this power. Every effort has been made to ensure these guidance notes are as helpful as possible.
Hua Deng, Ph.D., is the president and the founder of Davidia Healthtech LLC (davidiahealthtech.com), a professional service company for food, dietary supplement and cosmetics. Deng holds a doctorate from Lanzhou University in China with a major in analytical chemistry. She has extensive experience with global regulatory affairs in the food, dietary supplement and cosmetic industries.
About the Author
You May Also Like