Reporting and Analyzing Serious Adverse Events
Any manufacturer, packer or distributor whose name appears on the label of a dietary supplement marketed in the United States is required to submit any serious adverse event reports (SAERs) they may receive associated with use of the product in the United States.
July 13, 2018

Any manufacturer, packer or distributor whose name appears on the label of a dietary supplement marketed in the United States is required to submit any serious adverse event reports (SAERs) they may receive associated with use of the product in the United States (Section 761(b)(1) of the FD&C Act (21 U.S.C. 379aa-1(b)(1)). This requirement can sometimes be overlooked when implementing cGMPs (current good manufacturing practices) for dietary supplements, as it is covered separately by the Dietary Supplement and Nonprescription Drug Consumer Protection Act of 2006. As the cGMPs have additional requirements for handling complaints, both regulations must be considered when auditing for compliance.
A serious adverse event (SAE) for a dietary supplement is defined as an adverse event that:
Results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, or a congenital anomaly or birth defect; or
Requires, based on a reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above.
As soon as minimum data elements required for reporting to FDA (i.e., identifiable patient, initial reporter, identity and contact information of responsible person, suspect dietary supplement and SAE) are known to the responsible person, the 15-business-day time clock begins to run. The event must be reported to FDA using the MedWatch form (or electronically) within those 15 days. The Dietary Supplement and Nonprescription Drug Consumer Protection Act does not apply to foods other than dietary supplements. However, the FD&C Act does require a “responsible party” to inform FDA of “reportable food,” which is defined as an “article of food (other than infant formula or dietary supplements) for which there is a reasonable probability that the use of, or exposure to, such article of food will cause serious adverse health consequences or death to humans or animals.”
Implementation
All personnel involved in customer service and quality control (QC) must be trained to recognize and distinguish a SAE complaint from other product complaints, such as those involving defects or incorrect orders. On a routine basis, an individual with required training and expertise must review all complaints to ensure serious events are reported to FDA, if appropriate, and all manufacturing defects are investigated along with related batches.
Instructions for handling SAERs must be included in written standard operating procedures (SOPs); this is often best combined with regular returns and complaints handling SOPs. Using forms for customer service personnel to record complaints that include appropriate fields to collect the data required for SAERs is advised, along with approval by QC regarding whether the complaint should be reported to FDA.
FDA inspectors will review product complaints during an inspection, and may flag any events that, in their opinion, constitute SAEs. Report these events immediately, launch an investigation and corrective action plan, and make sure FDA is notified of the actions taken. As with any cGMP-related action, keep good records of product returns and complaints to ensure a complete paper trail.
Reporting an adverse event to FDA does not constitute an admission that a product caused or contributed to the reported event. FDA uses the reports in aggregate to identify trends or safety issues that may be related to products or their ingredients, and it’s important for individual firms to contribute to this body of evidence.
FDA Inspection Citations: Failure to Report
About 30 dietary supplement firms have been cited to date for failing to report SAEs when required. Additional related observations are for incomplete submissions and/or late submissions, or not using the correct form. For some reason, there was a larger number of failures to report during fiscal year (FY) 2012.
Title: Citations for Noncompliant Adverse Event Reporting, 2009-2017
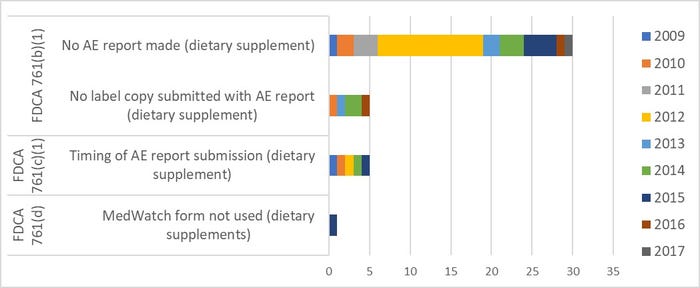
Source: FDA.gov
In addition to citations for failing to report SAEs, many firms have been cited for failures to adhere to cGMPs for complaints found during FDA inspections.
Title: Number of cGMP Citations Involving Failure to Properly Implement Complaints Procedures, 2009-2017
Source: FDA.gov
Title: Most Common cGMP Violations Related to Product Complaints, 2009-2018
Citation # | Frequency |
|---|---|
21 CFR 111.553 Written procedures - product complaint | 311 |
21 CFR 111.570(b)(1) Written procedures - product complaint; review, investigate | 197 |
21 CFR 111.560(a)(2) Product complaint - quality control investigate | 49 |
21 CFR 111.570(b)(2)(ii)(F) Record - product complaint; findings | 33 |
21 CFR 111.135 Product complaint - quality control review | 20 |
Source: FDA.gov
OpenFDA Data Portal
FDA publishes adverse events information using its free OpenFDA resources. The information in these reports has not been scientifically or otherwise verified as to a cause and effect relationship and cannot be used to estimate incidence (occurrence rate) or to estimate risk. However, certain trends are visible when analyzing the data. Caution must be taken not to overinterpret the data, as a report of an SAE does not establish cause and effect, and a single report may contain multiple food and dietary supplement products consumed, of which any, or none, might have contributed to the adverse reaction. Some examples follow.
Title: SAERs for Females, 2004-2017
Source: openFDA.gov
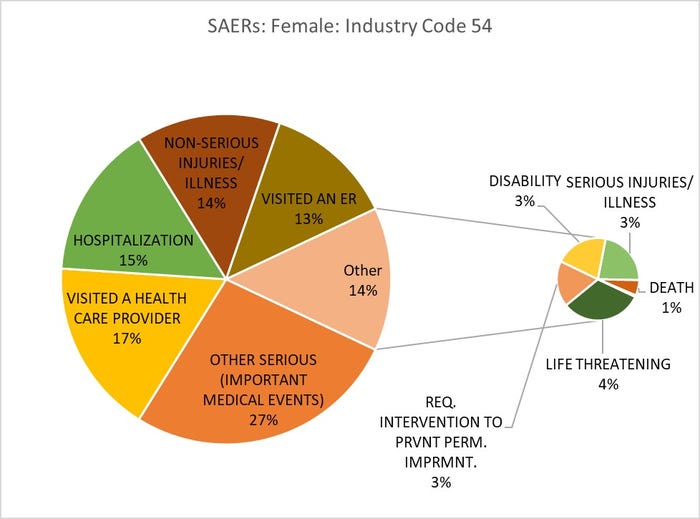
Title: Adverse Event Outcomes for Females Consuming Vitamins, Minerals, Proteins and Unconventional Diet (Human/Animal Foods)
Source: openFDA.gov
Marian Boardley is an independent consultant who manages projects for dietary supplement manufacturers and distributors. She helps clients in the food, drug and dietary supplement industries to implement compliance with dietary supplement, food and other cGMPs (current good manufacturing practices). She regularly trains staff to be ready for FDA inspections, and also writes documentation for quality control (QC), laboratory and manufacturing operations. Boardley was born in Yorkshire, England, and is now a U.S. citizen residing on the Colorado Plateau.
About the Author
You May Also Like