NIH Dietary Supplement Label Database (DSLD) is robust public resource
Officials at the National Institutes of Health explain the history of the Dietary Supplement Label Database, its functions and who's using it.
April 7, 2022
by Joseph M. Betz, Leila G. Saldanha, Johanna T. Dwyer and Richard A. Bailen
The National Institutes of Health (NIH) Dietary Supplement Label Database (DSLD) is a public database funded, developed and maintained by NIH’s Office of Dietary Supplements (ODS). The DSLD resulted from a directive from Congress in 2004 to develop, create, regularly update and maintain a collection of all labels from dietary supplements sold in the U.S. Today, the DSLD contains the labels of more than 136,000 dietary supplement products either currently or previously sold in the U.S. The DSLD, which captures the image of each label and all the text information printed on it, is available for free at https://dsld.od.nih.gov.
A prototype of the database was demonstrated to federal officials in 2008, and the first public release of the database was deployed in 2013 with 17,000 labels. Congressional encouragement prompted development of a mobile version of the DSLD, which was launched in 2017. The database software source code and graphical user interface was updated and modernized from 2020 to 2021, resulting in a redesigned DSLD deployed in August 2021.
The DSLD has an extensive set of search functions, enabling users to search for a specific dietary ingredient or for any word or phrase printed on a supplement label. The home page provides a prominent search bar and counter that displays the number of searchable labels in the database. Users can filter the labels and search results with increasing granularity. For example, one can select and narrow down the years when the labels were added to the database, and search by criteria such as product status as on market or off market, ingredient category, type of product, product form, intended target group and health-related claims. For specific ingredients, the DSLD links to sources such as the ODS dietary supplement fact sheets and targeted PubMed searches. Users can also download search results for further analysis and publications citation. An option is also available to download the entire database.
ODS retains two vendors to manage, maintain and populate the DSLD. Abt Associates, the original developer of the database, furnishes technical support for its programming and design. Therapeutic Research Center provides the labels. Labels are obtained and submitted for entry into the DSLD primarily by product manufacturers and marketers at no cost to them through a voluntary submission program called Manufacturers Connect. Instructions for submitting labels are provided on the DSLD website. The labels in the DSLD are updated regularly.
The DSLD captures the images and all information on the labels of dietary supplements. Because the manufacturer or distributor is responsible for this information, the labels might be incomplete, inaccurate or contain ingredients of concern to FDA. The DSLD website provides links to information on dietary supplements from ODS and from other government sources—for example, to the FDA Health Fraud Product Database. The inclusion of a product and its label in the DSLD is not an endorsement of that product by ODS.
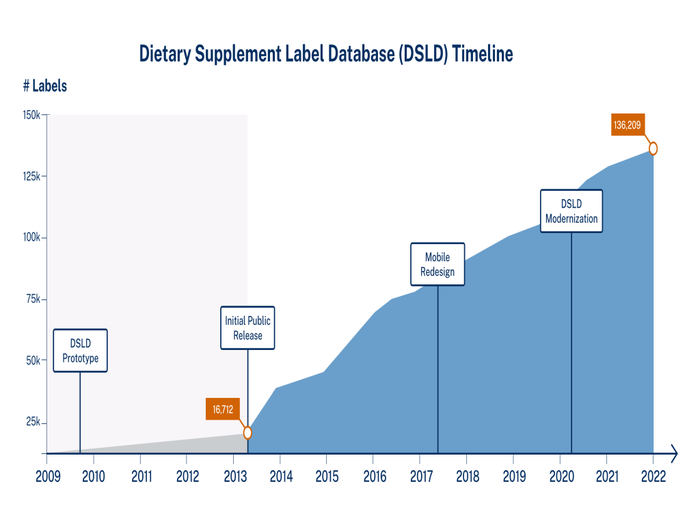
Dietary Supplement Label Database timeline showing label collection growth from 2009 to 2022, starting with 2009 prototype, and including the initial public release in 2013 containing 16,712 labels, mobile redesign in 2017, DSLD modernization in 2020, and present-day content of 136,209 labels.
How DSLD is being used
ODS has learned from Google Analytics reporting that half of DSLD users return to it repeatedly. Over 200 scientific papers have been published that use or cite the DSLD, including a study by investigators at the Uniformed Services University of the Health Sciences who used the DSLD to identify labels listing ingredients of concern in 49 products marketed for brain health and 18 for body building.1
One of the most common uses of the DSLD is by scientists to find the number of products with a specific ingredient or to look up an ingredient for their analytical methods development or toxicology studies. Departments of informatics, medicine and pharmacology at various colleges and universities are trying to link the DSLD with their medical and drug databases. FDA also uses the DSLD for analytical methods development and identifying products with ingredients that may have safety concerns.
Because the DSLD is used by researchers who may wish to study supplement use in a selected population group, labels are not removed from the database when a product is no longer marketed or is reformulated by the manufacturer. Old labels are retained but flagged with an off-market category code. The availability of off-market labels allows researchers to correlate data collected in past research (e.g., supplement use information collected by the National Health and Nutrition Examination Survey [NHANES]) with the labels of the actual products available at the time of the study. Off-market data also allows researchers to track changes in product formulations over time. Furthermore, having historic labels in the database has been useful to users to look up when a product was added to the DSLD.
ODS has also published several papers using information from the DSLD. One study examined the formulations of prenatal dietary supplements and found they often contain forms of iron not clinically studied to support iron nutrition during pregnancy (such as ferrous fumarate instead of ferrous sulfate).2 Label data also indicate these products often contain too much folic acid or contain a form of folic acid, L-5-methyltetrahydrofolate, which has not been proven to prevent the development of neural tube defects in a developing embryo.3 Another study evaluated 288 multivitamin-mineral products in the DSLD for children 1 to 4 years of age and discovered that while they provided an average of 10 vitamins and five minerals, only half of them contained calcium and only 60 contained potassium, two nutrients of public health concern.4
ODS regularly helps researchers and others who seek assistance on using the DSLD by responding to requests it receives through the Contact Us link in the database. Many of these requests come from professionals in the dietary supplement industry. Application developers also contact ODS to obtain the entire dataset of the DSLD, so a webpage on the site provides the API (application programming interface) download guide.
The DSLD remains a work in progress. In addition to regularly adding new labels to the database, ODS works to improve the user experience. Updates to the database can be tracked under the Release History tab. New versions of the DSLD will be more visually attractive, easier to navigate and provide improved search and download functions. Over time, we also hope to integrate the DSLD with other government food, nutrition, health and medicinal databases. Please contact us with any ideas you have for making the DSLD a more useful tool for you.
Joseph M. Betz, Ph.D., is acting director of the National Institutes of Health’s (NIH) Office of Dietary Supplements (ODS). Leila G. Saldanha, Ph.D., RD, is scientific consultant (contractor), ODS; Johanna T. Dwyer, D.Sc., RD, is senior nutrition scientist (contractor), ODS; and Richard A. Bailen, MBA, MHA, is senior program analyst, ODS.
References
1 Scott JM et al. “Using the Dietary Supplement Label Database to identify potentially harmful dietary supplement ingredients.” Nutr Today. 2018;53:229-233.
2 Saldanha LG et al. “The chemical forms of iron in commercial prenatal supplements are not always the same as those tested in clinical trials.” J Nutr. 2019;149:890-893.
3 Saldanha LG et al. “Perspective: Time to resolve confusion on folate amounts, units, and forms in prenatal supplements.” Adv Nutr. 2020;11(4):753-759.
4 Dwyer JT et al. “Do multivitamin/mineral dietary supplements for young children fill critical nutrient gaps?” J Acad Nutr Diet. 2022;122:525-532.
You May Also Like