Lab questionnaire guides contract lab audit
A lab questionnaire, a set a specific questions to ask a contract lab, can help a supplement brand choose the best testing partner to ensure GMPs, safety and efficacy.

In a previous article titled, "Picking the Right Contract Labs for Testing Success," I brought to light some of the many aspects and considerations involved in choosing the best fit for cGMP (current good manufacturing practice) testing needs. The article explained why a supplement brand might decide to outsource lab testing as well as various factors involved in making a decision to send test samples to a specific contract lab. Ultimately, I suggested not to have one lab, but rather have several labs based on their strengths and specialties. In this article, I will extend this conversation to what to do now that you have narrowed your choice of labs down to a select few. It’s time to audit.
As a contract manufacturer, the cGMPs are rules to live by. The industry has now had plenty of time to take a big gulp of this reality and swallow it down. Federal laws state a supplement brand must confirm the identity of all materials used in a product using scientifically valid methods. Choosing a contract lab based on price is an activity commonly known as "lab shopping," and can produce undesired results.
This reminds me of a German mechanic that cared for the 1984 Volkswagen van I drove in high school. Next to the cash register sat a large, brittle plastic sign that read, “The bitterness of poor quality remains long after the sweetness of low price has GONE." Back then, this was something I got to read as they drained my bank account, like punishment for doing the right thing. Later, I find this saying applies to many things in life and business, and that quality really matters.
While there are not necessarily many labs to choose from in this industry, there are certainly enough to create a full-time position just for evaluating their strengths, weaknesses, specialties, reputation, potential conflicts, turn around time and price. Price should be one of the last factors used for determining which lab you choose. "I’m glad we saved money on those cheap lab tests," said no one ever while being audited by FDA.
A contract lab is an extension of the contract manufacturer, and cGMPs apply to them too. The same vendor qualification process used when determining a vendor for key supplies or ingredients must be mimicked when picking a lab. This is done by auditing the lab.
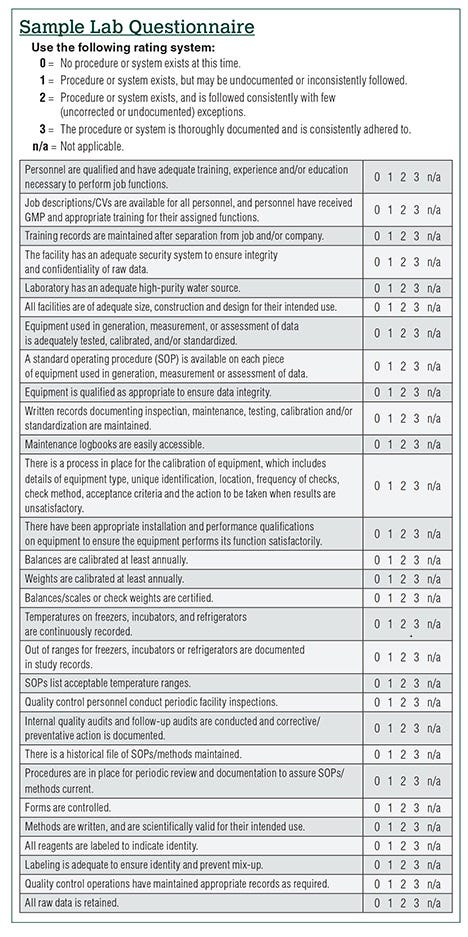
As a lab that commonly leverages the strengths of our "cooperative rivals" to facilitate testing that is not performed in house, Alkemist Labs settled on only a small handful of labs that we feel are qualified and trustworthy. We determined such by first sending a lengthy, arduous and detailed lab questionnaire. This questionnaire is a mini audit that goes over numerous aspects from the quality system to validation of test methods and staff training. It is simply not fun to fill it out, and many labs have either refused to complete it or inadequately filled it out. These labs are not on our approved lab list.
Some labs required a "non-compete agreement" prior to filling out our lab questionnaire. This too was a deal breaker, and such lab is not used.
Labs should have nothing to hide, especially from each other. There should be no proprietary methods, hidden data or secret results unless it is particular for a custom product. Test methods should be available in full. If a contract manufacturer is to use a contract lab to prove to FDA that what is on the label is actually in the package, then they better have the data in full to back up claims.
Auditing a lab is a time-consuming, expensive process. Generally, it’s a one- to two-person operation and requires air travel, an overnight stay in hotels and other travel-related expenses. It is not uncommon that we get audited a few times a month by our customers (contract manufacturers as well as from other labs). Surprisingly, the extent at which we are audited spans a wide range of superficial oversight making sure a real lab exists and has equipment, to a fully engaged audit where lab notebooks are scoured for missing signatures and dates or calculations performed incorrect.
A company can also send a qualified staff member to see the lab for themselves. An in-person audit is irreplaceable, however, not always possible. A lab questionnaire can do a great job, but still relies on trusting that "what is on the questionnaire is actually happening in the lab." This is why choosing a few select labs to work with and maintaining the relationship is critical to the success of your cGMP compliance.
Critical data to request in a lab questionnaire
Request the following documents:
Organizational chart
Facility floor plan
Laboratory certifications/accreditations
Instrument list (including detectors)
Process flow chart (identifying critical stages and points of testing and reporting)
Standard operating procedure (SOP) table of contents
OOS procedure
Training procedure
Sample/chain of custody procedure
Sample submission form/chain of custody
Standard turn around times along with associated rush charges.
Fee schedule for data packets & investigations
Service agreement
Price list including: test, test method reference, instrumentation used
Élan M. Sudberg is CEO of Alkemist Labs, an analytical testing lab. He holds a degree in chemistry from California State University Long Beach, and has authored numerous journal articles on phytochemistry and analytical techniques for the natural products and nutraceutical industry. He is a newly appointed board member of AHPA’s Education and Research on Botanicals Foundation and is the chair of the Hemp and Medical Marijuana committee.
About the Author
You May Also Like