Essential Minerals for Heart Health
The heart consumes more energy per gram than any other organ in the body, so adequate amounts of chemical fuel, adenosine triphosphate—or ATP—must be generated to support the approximately 100,000 heart beats that pump about 10 tons of blood per day. There is a very close correlation between supply and demand to keep up with the 10-fold increase in energy requirements during exercise, but even at rest, the heart cycles about 6 kg of ATP per day (20 times its own weight). Magnesium plays a key role in that achievement.

Amazingly, the heart consumes more energy per gram than any other organ in the body.1 Adequate amounts of chemical fuel, adenosine triphosphate—or ATP—must be generated to support the approximately 100,000 heart beats that pump about 10 tons of blood per day. There is a very close correlation between supply and demand to keep up with the 10-fold increase in energy requirements during exercise, but even at rest, the heart cycles about 6 kg of ATP per day (20 times its own weight). Magnesium plays a key role in that achievement.
What exactly is ATP?
ATP is a molecule that stores and distributes energy in perfect amounts exactly when your body needs it. But how is that energy packaged and delivered?
Chemical energy is primarily stored in the phosphate bonds of substances like ATP. These high-energy phosphate bonds have been compared to the cells of a battery, and in a sense, are the ‘currency’ with which work gets done in every cell in the body. ATP is the most important of these phosphate-containing energy substances and its high demand makes it absolutely essential for normal function of the heart muscle. As a result, ATP turnover is many times the size of the ATP pool maintained by the body. Therefore, ensuring this extremely high demand for energy is so critical that the heart muscle cells (myocytes) are capable of converting a variety of carbon-based fuels into ATP the heart needs.
Supply and demand
What does supply and demand have to do with energy in the heart? The extremely high and unrelenting energy requirements of the heart have made it imperative that its energy is supplied by more than one source to prevent a catastrophic outcome should a single source suddenly be stopped or slowed. It is easy to compare the dependence of a large economy on only one source of crude oil, for example. How much more vulnerable would the United States be if all its oil came from one country which could be threatened by political instability or natural disaster? It is for this reason that the heart’s energy supply minimizes its risk by ensuring multiple metabolic pathways to ensure a steady supply.
How does ATP work?
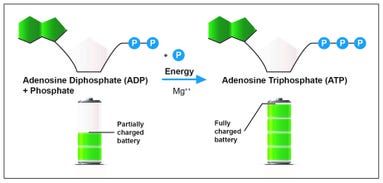 The characteristics of ATP make it an exceptionally useful molecule that is used by all types of cells as their basic energy source.4 An ATP molecule consists of a nitrogen-containing compound called adenine, a five-carbon sugar called ribose, and three phosphate groups. Adenosine diphosphate (ADP) has a similar structure to ATP, but with one important difference: ADP has two phosphate groups instead of three. This difference is the key to the way in which cells store energy. When a cell has energy available, it can store small amounts of energy by adding a phosphate group to ADP molecules, producing ATP molecules, as shown in Figure 1. This is where the analogy to a battery cell comes in. ATP represents the fully charged form of the molecule, ready to power the machinery of the cell. The energy stored in ATP is released when it is converted into ADP. Because a cell can add and subtract a third phosphate group, it has a way of storing and releasing energy as needed! ATP carries just enough energy to power a variety of cellular activities.
The characteristics of ATP make it an exceptionally useful molecule that is used by all types of cells as their basic energy source.4 An ATP molecule consists of a nitrogen-containing compound called adenine, a five-carbon sugar called ribose, and three phosphate groups. Adenosine diphosphate (ADP) has a similar structure to ATP, but with one important difference: ADP has two phosphate groups instead of three. This difference is the key to the way in which cells store energy. When a cell has energy available, it can store small amounts of energy by adding a phosphate group to ADP molecules, producing ATP molecules, as shown in Figure 1. This is where the analogy to a battery cell comes in. ATP represents the fully charged form of the molecule, ready to power the machinery of the cell. The energy stored in ATP is released when it is converted into ADP. Because a cell can add and subtract a third phosphate group, it has a way of storing and releasing energy as needed! ATP carries just enough energy to power a variety of cellular activities.
What role does magnesium play?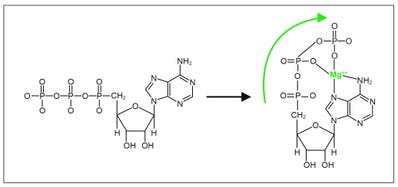
Energy is a physical force, not a chemical compound. Energy can be stored as chemical potential energy in the electronic configuration of a “high energy" bond, a unique bonding of phosphorus (P) and oxygen.
Because of its +2 charge, magnesium (Mg) has the ability to electrically interact with the phosphate tail of ATP (negative charge). Magnesium anchors on the nitrogen groups of adenine and ‘pulls’ the tail of the phosphate chain toward the adenine molecule and creates a large strain on the bond between the second and third phosphate group. There is some strain in the bond between the first and second phosphate molecules, but much less so.
Therefore, it is in this terminal phosphate bond that most of the kinetic energy of ATP is stored—just like when a bow string (as in a bow and arrow) is pulled back, and the further the string can be drawn, the higher the kinetic energy will be released to the arrow upon release of the string. In the ATP molecule, the storage of energy by adding one more phosphate group to ADP (adenine diphosphate) requires magnesium to bind phosphates two and three to the nitrogen atoms of the adenine molecule.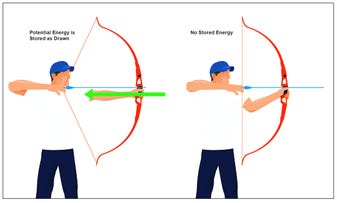
With that finite ‘parcel’ of energy, the magnesium-phosphate bond is enzymatically broken, thereby releasing a phosphate molecule and a defined amount of energy. You could say that ATP is the energy currency of all biologic cells.
Magnesium is energy’s secret weapon
Magnesium is needed to ‘charge’ the ATP battery—and its enzymatic separation from ATP is what releases energy. Magnesium’s value to the heart’s health is crucial, but it is also crucial to the vitality of all cells, which is why it is the key co-factor in more than 300 metabolic reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control and blood pressure regulation.5,6,7 Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm.
There are many studies showing a significant correlation between people with low levels of magnesium who demonstrate a higher incidence of heart attack and stroke. Dietary surveys of people in the United States consistently show that intakes of magnesium are lower than recommended amounts. Habitually low intakes of magnesium induce changes in biochemical pathways that can increase the risk of illness over time.8
Not all magnesium is the same; organic magnesium forms such as magnesium glycinate chelate are recognized as more bioavailable and also tend to cause less gastric upset. Providing all body tissues, especially the heart, with optimal levels of this remarkable mineral often requires making up for an inadequate dietary intake. Choosing a quality magnesium supplement couldn’t be more important.
Jonathan Bortz, M.D., earned his medical degree from the University of the Witwatersrand Medical School in Johannesburg, South Africa. In 1995, he founded the Bortz Diabetes Control Center—a full-service, integrated, multidisciplinary diabetes clinic and clinical research center. He worked in private practice as an endocrinologist for 15 years before entering the pharmaceutical industry. He has served for many years as a medical affairs consultant to Albion Minerals and is now the chairman of Albion’s scientific advisory board and director of scientific commercial development.
References:
1. Weiss RG, Maslov M. Advanced Studies in Medicine. 2004:4 (6B) S457-463.
2. Ingwall JS, Weiss RG; Circulation Research. 2004;95:135-145.
3. Doenst T et al. Circulation Research. 2013;113:709-724.
4. Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997.
5. Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare; 2010:527-37.
6. Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins; 2012:159-75.
7. Moshfegh A, Goldman J, Ahuja J, Rhodes D, LaComb R. 2009. What We Eat in America, NHANES 2005-2006: Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium. U.S. Department of Agriculture, Agricultural Research Service.
About the Author
You May Also Like