Natural or SyntheticHow Do You Know?
Testing the authenticity of claims an ingredient is natural or bio-based is the basis for new proposed analytical standards from the USP.
February 10, 2011

by James C. Griffiths, Markus Lipp and Jeffrey C. Moore
Consumers demand more from manufacturers. Its a fact:
A dietary supplement must be economical (a relative measure).
A dietary supplement must be safe (relative).
A dietary supplement must be efficacious (relative).
Now, a dietary supplement must be natural (absolute).
This last demand is a marketers dreamor nightmare. Todays savvy customer wants something with desired effects, without collateral damage to her wallet, as well as assurance that the environmental impact is minimized (i.e., renewable). Further, there is a growing perception that somehow safety and efficacy are tied to a products origin, with natural deemed better than synthetic. The dietary supplement industry is facing these market demands most notably in the area of vitamins, in particular vitamins A and E and folic acid, as well as some extracted compounds such as resveratrol, chondroitin and L-theanine.
Before obsessing on philosophic nuances regarding natural, renewable, environmental footprint and the like, we need to define a few terms for the sake of this discussion. This article addresses organic, carbon-based materials. So natural in this context means materials from current plant or animal sources. Synthetic will refer to ingredients that have been sourced or derived from crude oil, coal or any product made thereof, such as petroleum, ethylene, benzene, mineral oils, etc.
It is interesting to note synthetic is natural in the sense that it has sat for eons and all petrochemicals trace their existence to plant/animal-based carbon origins. Moreover, minerals can be natural, but in the context of the test method discussed here, which is based on carbon isotopes, we will refer only to ingredients or products that have used fresh and/or recently harvested animals/plants as their source. It makes more sense to use the following terms: Bio-based carbon versus petrochemical-based carbon; but, if it is easier for the reader, the shorthand natural and synthetic can be mentally visualized. Of course, the two are intrinsically linked and mutually exclusive, as any material consisting only of bio-based carbon will not contain any carbon from petrochemicals and vice versa.
A new method for determining the bio-based content of food ingredients and dietary supplements is among the latest proposed additions to the Food Chemicals Codex (FCC) compendium. All additions to FCC come through the FCC Forum, the free-access website through which the U.S. Pharmacopeial Convention (USP)the organization that publishes FCCaccepts public comment on proposed FCC standards.
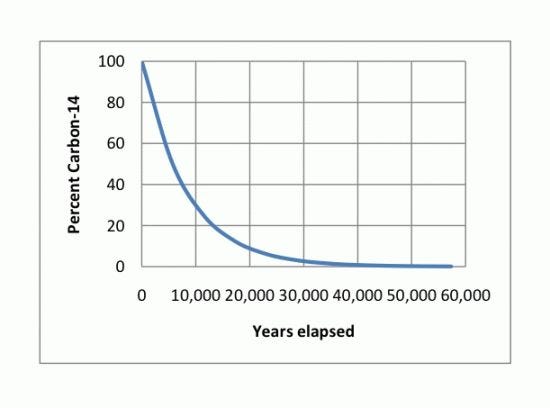
The method proposed for inclusion in FCC uses carbon isotope analysis to measure bio-based content. An understanding of carbon isotope chemistry is helpful to understand how this works. Carbon has three naturally occurring isotopes: two stable and one radioactive. Carbon-12 (C-12; 12C) is the most abundant of the stable carbon isotopes accounting for 98.89 percent of natural carbon, and has a nucleus containing six protons and six neutrons. Carbon-13 (C-13; 13C) is the other stable isotope and makes up about 1.1 percent of all natural carbon. It contains six protons and seven neutrons in its nucleus. Carbon-14 (C-14; 14C, or radiocarbon) contains seven protons and eight neutrons in its nucleus and is a radioactive isotope of carbon. Carbon-14 naturally occurs in trace amounts in the environment, making up as much as one part per trillion (0.0000000001 percent) of the carbon in the atmosphere. Carbon-14 decays with a half-life of 5,730 ±40 years into nitrogen-14 (14N). For example, a plant material that is 5,730 years old would be expected to contain half of the amount of radiocarbon when compared to a modern plant equivalent. The dating of archaeological, geological and hydro-geological samples containing organic matter is often carried out with this principle using radiocarbon dating. Living plants and recently harvested plants and animals will have radiocarbon in them in an amount defined as 100 percent. Petrochemicals, in contrast, are the product of plants and animals that died hundreds of million years ago and contain only an infinitesimal amount of radiocarbon (i.e., 0 percent) because it has long since decayed away (Figure 1).
The FCC-proposed method to determine bio-based content calls for analyzing samples by first completely combusting them to convert all carbon quantitatively to CO2, followed by the use of accelerator mass spectrometry to determine the distribution of carbon isotopes. This analysis is carried out on both a sample and a reference standard (a recently harvested organic material of known age), and the ratio of carbon isotopes (either 12C:14C or 13C:14C) from both the sample and reference standard are used to calculate bio-based content. A value of 0 percent indicates all carbon originated from petrochemicals. A bio-based content value of 100 percent indicates that all carbon originated from modern plants or animals.
The method proposed in FCC is suggested for the bio-based content analysis of 1,3-propanediol (a new food ingredient), but is suitable for analysis of all carbon-based materials. It therefore is applicable and maybe useful to all industries needing to verify a claim that an ingredient is derived from current bio-based materials rather than crude oil. For dietary supplements such as vitamin E, beta-carotene or folic acid, the carbon isotope analysis method could be used to verify that materials are indeed of the origin claimed. This technique can also be used for counterfeit detection, as in detecting the fraudulent addition of a synthetic compound to an expensive natural extract. For example, the new method would be able to detect the addition of synthetically produced vanillin to natural vanilla extractsomething that other anti-counterfeiting methods are not specific enough to do.
USP intends to expand the FCC appendix on authenticity methods in the future to include additional procedures for detecting counterfeit food and/or dietary supplement ingredients and is encouraging industry to submit useful methods for consideration.
The carbon isotope analysis method proposed here was originally published by the American Society for Testing and Materials (ASTM), a globally recognized standards-setting organization and leader in the development and delivery of international voluntary consensus standards. As is typical for materials included in FCC, industry stakeholders approached USP with the request of adding the ASTM method to differentiate synthetic from bio-based materials. The method is set to be republished in FCC after the public comment period ends, and its suitability is verified by USPs Food Ingredients Expert Committee, a group of volunteer experts who evaluate proposed standards based on current science.
The carbon isotope analysis method is a new resource to all parties, buyers and sellers alike, that has been previously unavailable in FCC. The method is suitable for the verification of a claim that a material is bio-based, which is otherwise difficult for buyers to verify or for sellers to demonstrate. The proposed method fits the overall model of FCC to not only make quality standards for the authenticity, quality and purity available, but also to supply all parties in the supply chain with suitable means for verification of bio-based food ingredients and accompanying reference materials to demonstrate the methods have been performed appropriately.
The proposed additions to FCC support USPs mission to improve the health of people around the world through public standards and related programs that help ensure the quality, safety, and benefit of medicines and foods. By including FCC among its core compendia, USP is advancing the goal of making it the leading international resource for food and dietary supplement manufacturers, providing world-class methods and emphasizing its support of innovative and widely used food ingredients. With its standards designed to enable all parties to verify the authenticity, purity, and quality of ingredients throughout the food and dietary supplement supply chain, FCC helps in maintaining the integrity of an ingredient as it moves through the supply chain and global markets. Through its FCC Forum, USP asks all manufacturers and other stakeholders to review these proposed standards and share their valuable feedback with USP. USP is always interested in working with industry to develop new standards for inclusion in future editions of FCC.
The method, titled Appendix XIV: Markers for Authenticity Testing, is currently published and available for public comment until March 31, 2011. Food or dietary supplements manufacturers, their suppliers and all other interested parties are invited and encouraged to provide comment on the latest proposed FCC standards available on the FCC Forum which is accessible at USP.org/FCC/FCCForum.html.
Dr. James Griffiths is Vice President of Food, Dietary Supplement and Excipient Standards at the U.S. Pharmacopeial Convention (USP), and holds a Ph.D. in Toxicology. Dr. Markus Lipp is USP Director of Food Standards, and holds a Ph.D. in Analytical Chemistry. Dr. Jeffrey Moore is USP Scientific Liaison for Food Ingredients, and holds a Ph.D. in Food Science. All three scientists lead efforts to identify quality standards and methods for inclusion in USP's Food Chemicals Codex.
You May Also Like