Consumers can’t rely on FDA to adequately target ‘bad actors’ in supplements market
Since more than 70 percent of Americans use supplements, it’s time to explore alternatives to FDA’s regulating the marketplace.

This is part of Insider’s periodic updates from trade associations and other major stakeholders. These are meant to keep industry readers updated on these organizations’ activities and priorities, as well as to issue calls for action. These are op ed commentaries and represent the views from the authors and/or the organizations they represent, not the views of Insider or its editorial staff.
It is my view that FDA is not adequately enforcing existing laws/regulations involving the US$55 billion-a-year supplement industry. And that could be putting consumers at risk.
Is the agency adrift? It has not had a permanent commissioner for over a year. There has been a rotating door of ‘acting directors’ within the FDA Office of Dietary Supplement Programs (ODSP) ever since attorney Steve Tave moved elsewhere within the agency in March 2021.
Issuance of final guidance affecting new dietary ingredients (NDIs) has been stalled for years. Following a lengthy public hearing years ago, as well as submission of stakeholder testimony and congressional pressure, FDA still has not established a clear regulatory pathway for CBD and other cannabinoids. Further, the agency has not addressed citizen petitions seeking clarity regarding the ability of companies to continue to market supplements containing NAC (N-acetyl-L-cysteine). And during the pandemic, the frequency and number of inspections for compliance with cGMPs (current good manufacturing practices) has declined.
FDA seems rudderless and unable to fulfill its statutory mandate—this could put consumers at risk and place responsible supplement and ingredient companies at a competitive disadvantage. Somewhat in jest, this reminds me of an opinion once expressed by President Ronald Reagan:
"The closest thing to eternal life on earth is a government program."
I began having concerns as to FDA’s ability to adequately enforce existing laws and regulations in 2016 when I was with Boise-based Bodybuilding.com (BBCom). That year, BBCom’s Chief Legal Officer Bill Carter, its Product Regulatory Specialist Andy Swanson (now with NutraBolt) and I met in Seattle with FDA’s District Director and her staff. The purpose of our visit was to familiarize FDA with BBCom’s enhanced regulatory compliance systems used in on-boarding and promoting third-party brands. Additionally, we shared concerns with FDA about the scores of violative products then available for sale on Amazon. Literally, it was years before some enforcement action was taken.
Earlier this month, Bill Carter, former BBCom compliance staffer Jason Hendrickson and I were discussing two potentially problematic products marketed as supplements:
Xanrelax contains an extract of the botanical commonly known as kratom. XanRelax Capsules | Anti-Anxiety + Euphoria | No Prescription Needed – K-Chill Direct (kchilldirect.com)
Addall XR Brain Boost Supplement 750 mg/Capsule - 12 Pack (24 Capsules) - Ships in a Box + Free Pack of Vitamin B12 Pills (4 Capsules) https://www.amazon.com/dp/B0844XTFP6/ref=cm_sw_r_cp_api_glt_fabc_GJGE78J1298DW0PXQ0B7
Pic is from an Amazon review. Supplement products containing 2-amino-6-methylheptane (known as DMHA) are adulterated, according to FDA. https://www.fda.gov/food/dietary-supplement-products-ingredients/dmha-dietary-supplements).
This product is still available on Amazon as of the time of this article being written, however DMHA is no longer listed on the Supplement Facts panel. (An historical image of the product label is below.)
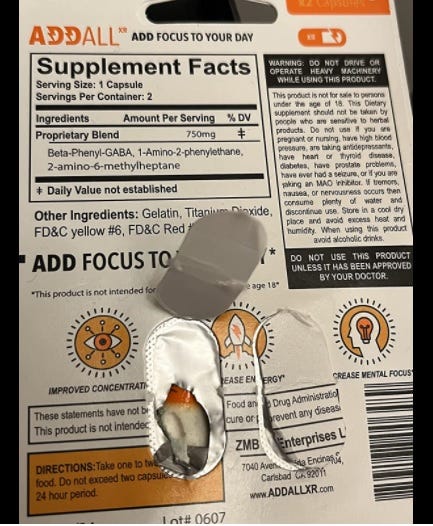
Historical label shot of Addal, posted to Amazon.com on Nov. 11, 2021 by user JediSmitt
The fact that illegal “supplements” have been found on Amazon speaks volumes about the weakness of the retailer’s “enhanced” onboarding and regulatory compliance capabilities.
What can be done?
Three years ago—on February 11, 2019—then FDA Commissioner Scott Gottlieb, M.D., who uses supplements, unveiled a multi-pronged plan to modernize how FDA regulates such products.
Dr. Gottlieb said: “We know most players in this industry act responsibly. But there are opportunities for bad actors to exploit the halo created by quality work of legitimate manufacturers to instead distribute and sell dangerous products that put consumers at risk.
“As the popularity of supplements has grown, so have the number of entities marketing potentially dangerous products or making unproven or misleading claims about the health benefits they may deliver.”
Some will say no new regulations or laws are necessary. Instead, FDA, should do a better job enforcing existing provisions while going after so-called bad actors. As NPA President and former FDA-er Dr. Dan Fabricant noted, “Why are we talking about modernizing something that has never been fully implemented?” Indeed, ODSP has about three dozen personnel responsible for enforcing regulations affecting more than 2,000 firms and 80,000 products. Clearly, ODSP lacks the resources to protect the public’s health while ensuring a level regulatory playing field.
Consolidating food policies under one roof
As supplements are regulated as food in the U.S., I suggest the Biden administration and Congress consolidate all of America’s food policy activities under one roof. The idea would be to move the Center for Food Safety & Applied Nutrition (CFSAN) plus ODSP from FDA into an enhanced U.S. Department of Agriculture & Nutrition (USDAN). USDAN would have jurisdiction over all U.S. food and nutrition policy, regulation and enforcement.
Further, let’s give FDA/USDAN the authority not only to seize violative products, but also to disgorge assets from offending parties. Seized assets could be used to add full-time employees to ODSP, so it could more effectively enforce current regulations.
I also recommend requiring USDAN to notify the Office of Inspector General (OIG) when the agency learns of potentially violative products from tips or inspections. Monthly, USDAN should disclose to OIG the status of investigations that should be completed in no more than one year following receipt of the initial complaint. Federal employees found to have not properly executed their responsibilities should be held accountable and be subject to demotion or termination for cause.
Engaging with plaintiffs’ bar
When I was at BBCom and since becoming a consultant, I have periodically emailed ODSP or FDA’s Office of Regulatory Affairs (ORA), directing them to potentially violative products. Some of these products contained drug analogues, others contained ingredients banned by FDA, while still others made inappropriate claims on labels. From a distance, it has been difficult to discern if the agency took any action. Consumers may well have been at risk.
In 2016, my former colleague Bill Carter introduced me to several proactive members of the plaintiffs’ trial bar. Over time, Bill and I have reached out to these attorneys to alert them to “supplements” containing illegal prohormones or drug analogues, products making inappropriate claims, products that when analyzed do not meet label claims and products that could be at risk of Proposition 65 violations. While the plaintiffs’ bar is not altruistic in its motivations, it poses one additional means by which companies can potentially level the regulatory playing field in the absence of FDA engagement.
Bottom line
For whatever reason, FDA has not fully utilized its existing authority to protect the public health from potentially dangerous products that do meet federal requirements. Therefore, responsible stakeholders may wish to consider alerting members of the plaintiffs’ bar who can initiate state or federal litigation to hold “bad actors” accountable. Secondly, considering over 70% of Americans take supplements—as well as FDA’s revolving door and lack of engagement— perhaps it is time for stakeholders, members of Congress and the current administration to consolidate all agriculture, food and nutrition regulatory, inspection and enforcement authority into an efficient Department of Agriculture and Nutrition.
John Venardos is a global regulatory and government affairs consultant with nearly forty years of experience working in 71 countries on behalf of The NutraSweet Company, Pfizer, Herbalife Nutrition and Bodybuilding.com. He has previously served in leadership positions in domestic and international associations. He serves on the board of RekemendRX and on the corporate advisory committee to SummaForte LLC. His clients include ingredient suppliers, CPG brands plus venture capital/private equity investors seeking due diligence assessments involving the natural products and direct selling industries. He can be reached at [email protected].
About the Author
You May Also Like