Supplement maker, FDA met several times over NDI enforcement
People close to nutritional supplements maker Natural Alternatives International Inc. met numerous times with FDA to persuade the agency to target an ingredient produced in China—beta-alanine—that hasn't been the subject of FDA review.
Editor’s note: This is part two of a four-part series of articles on FDA enforcement of the new dietary ingredient notification requirement in U.S. law. Part one can be read here.
On the evening of Feb. 26, 2019, Mark LeDoux, the founder of Natural Alternatives International Inc. (NAI), sent an optimistic email to some colleagues after meeting with FDA officials. Just weeks earlier, NAI—a nutritional supplements maker in Carlsbad, California—received word that FDA had acknowledged its safety-related notice for a new dietary ingredient (NDI) manufactured in Japan, CarnoSyn beta-alanine.
It took NAI nearly a year to compile all the information for its beta-alanine NDI notification (NDIN) to FDA—with hundreds of pages of documents describing such matters as testing, manufacturing methods and safety, LeDoux explained in an interview. NAI spent a minimum of around $1 million investing in the NDIN process, and the figure is more than twice that amount when including human clinical trials to support components of the submission, he reported in a follow-up email.
During the Feb. 26 meeting with FDA officials, LeDoux turned his attention to rival forms of beta-alanine. It would not be the last time NAI complained to FDA that beta-alanine coming into the U.S. from China was “adulterated” and possibly dangerous to consumers. Over a period of more than a year, NAI and its advocates communicated with FDA employees—including Steven Tave, director of the Office of Dietary Supplement Programs (ODSP)—through emails, phone calls and in-person meetings.
NAI believed it was making headway with FDA, its representatives suggested in emails following meetings with the agency. In recent months, though, FDA has declined requests for additional meetings by the head of a trade group with close ties to LeDoux, and the agency has not issued an import alert for “generic beta-alanine,” as NAI requested. FDA representatives suggested in an email and interviews that the agency� was not provided sufficient evidence to justify enforcement action against the ingredients flagged by NAI.
This article highlights the substance of NAI’s pleas to FDA, points at which NAI’s advocates seemed encouraged by meetings with agency officials, and the conclusions of the two parties following more than a year of back-and-forth conversations.
2019 meetings
On Feb. 1, 2019, FDA advised NAI’s outside counsel, Kevin Bell, that it filed NAI’s NDIN for CarnoSyn beta-alanine. The acknowledgement letter was welcome news for NAI, reflecting the culmination of a substantial investment and confirmation that the company had satisfied a requirement in the law to establish a supplement containing an NDI “will reasonably be expected to be safe.”
Twenty-five days later, LeDoux—chairman and CEO of NAI—met with FDA officials, including Tave. The objective, he recalled for this story, was “to discuss policing those products that were piggybacking on our successful NDI to import their beta-alanine without submitting safety or process data.”
The meeting had been productive, LeDoux suggested to Corey Hilmas, a medical doctor and former FDA official working for the Natural Products Association (NPA), in a Feb. 26, 2019 email sent that evening.
“I think we set the table for some productive outcomes based on relationship and the mutual recognition that FDA needs a quid-pro-quo for the NDI in order to establish the intrinsic commercial value of the undertaking for those companies that want to play by the rules,” LeDoux, who chairs NPA’s board of directors, wrote to Hilmas, according to a partially redacted email Natural Products Insider obtained from Bell, a partner in Washington, D.C., with the law firm Arnall Golden Gregory LLP (AGG).
Hilmas, who now works for KGK Science and was not immediately available to comment for this story, also attended the meeting.
In an earlier email that day to LeDoux, Hilmas indicated FDA expressed interest in the men drafting an import alert—which authorizes the detention of products (that appear to violate the law) at U.S. ports without physical examination—to support an adulteration charge. “Dan and I can work on that,” Hilmas said, referring to Dan Fabricant, president and CEO of NPA, who previously oversaw FDA’s Division of Dietary Supplement Programs.
That spring, LeDoux capitalized on another opportunity to discuss NDIs at a public meeting hosted by FDA, “Responsible Innovation in Dietary Supplements.”
During the May 16, 2019 meeting, Tave reminded industry that “an effective NDI notification process represents FDA's only opportunity to evaluate the safety of a new dietary ingredient before it becomes available to consumers.”
FDA’s “goal overall is not to [maximize] the number of notifications that we receive,” he said, according to a transcript of the meeting. “Rather, our goal is to right-size the process to see that appropriate notifications are submitted for the products for which they are required.”
Several people from industry spoke during the meeting, including LeDoux, who said his company “spent millions of dollars” and entered FDA’s “front door” by submitting an NDIN.
“Filing an NDI notification should not be considered too difficult; however, spending those kinds of resources as either a private or public company begs the question, ‘We’re a good citizen; now what?’” he stated.
“So by helping the government do its job, which is to promote the safety of consumer products in our space, we’re looking at ways to work together with the agency to arrest those products that are in commerce that I believe are deficient in not only scope, content, but are, in fact, per se, adulterated because they have not gone through the front door of the FDA,” LeDoux said.
NAI and its advocates would reiterate this ‘adulteration’ theme in various correspondence with FDA. In support of their requests that FDA take enforcement action against beta-alanine, Bell and Fabricant provided records to FDA pertaining to the ingredient.
A month after the public meeting, Bell and Fabricant met again with FDA officials, providing “extensive data regarding imports of beta-alanine” between 2017 and May 2019, according to a timeline of events Bell prepared for this story. During the June 14 meeting, FDA was asked to take action against entities violating the law, based on NAI’s NDIN.
Bell and Fabricant made clear the “entire industry was taking a ‘wait and see’ approach on buying CarnoSyn to see if the FDA was going to do anything,” Bell shared via email, in what he said reflected excerpts from a summary of the meeting. “If the FDA would take action, we believed it would have widespread effect on companies’ actions in being compliant.”
Later that year, in an email sent Oct. 4, 2019 to Tave and another ODSP employee, Sibyl Swift—now NPA’s senior vice president of scientific and regulatory affairs—Fabricant attached an excel spreadsheet of 10 supplement brands marketing beta-alanine and the beta-alanine products for each of the brands: Iovate, ProSupps, Redcon1, JNX Sports, MusclePharm, GHOST Lifestyle, Bulk Supplements, Old School Labs, Vital Pharmaceuticals and Bucked up.
Six of the companies began licensing CarnoSyn beta-alanine in either 2015, 2016 or 2017, though the last purchase by any of them (MusclePharm) was in March 2019, according to Bell. None of the brands responded to requests to comment for this story, whether on their legal basis for marketing beta-alanine in the U.S., or their reaction to NAI’s requests that FDA target for enforcement action beta-alanine that hasn’t been the subject of an NDIN.
Also on Oct. 4, 2019, in a separate email addressed to Frank Yiannas, FDA deputy commissioner for Food Policy and Response, Fabricant requested a meeting �“to discuss the absence of enforcement on imported new dietary ingredients, which have failed to file an NDI notification.” He advised FDA of an upcoming trade show (SupplySide West), where “the show floor is filled with imported knockoffs of NDIs.”
“No one at FDA has seen the specifications behind such products or the underlying safety data as is required by statute,” he wrote in the email, which also was sent to other FDA employees, including ODSP officials and Douglas Stearn, deputy director for regulatory affairs with the Center for Food Safety and Applied Nutrition (CFSAN).
“This creates a completely unbalanced playing field, effectively sending the message to U.S. companies that successfully submit an NDI that the agency is fine with someone claiming to have the exact same material as a company that submitted, without any evidence to show on that front.”
Four days later in an email to Yiannas, Fabricant attached for FDA’s consideration a draft import alert/bulletin for beta-alanine coming into the U.S. without an NDIN. It essentially proposed detaining products and bulk dietary ingredients containing beta-alanine that hadn’t been subject to an NDIN.
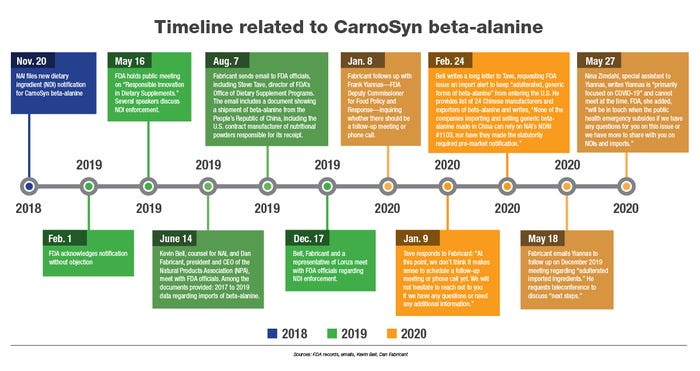
On Dec. 17, 2019, at FDA’s campus in White Oak, Maryland, Bell and Fabricant met again with FDA officials to discuss NDI enforcement. According to the two men, Yiannas appeared by phone while several officials appeared in person, including Stearn, Tave and Cara Welch, now deputy director of ODSP, who prior to her tenure at FDA worked at NPA. Also present, according to Bell and Fabricant, was a representative of Lonza, the multinational company with a specialty ingredients segment. Melanie Disa, a spokesperson for Lonza, did not respond to multiple requests for an interview to comment on NDIs.
The conversation related to the broader issue of import alerts, as well as specific companies and NDIs including beta-alanine, Bell and Fabricant confirmed.
While FDA officials expressed interest in the issues, “Steve Tave told us to be patient,” Fabricant said. “We had been sending information for over a year specific to the beta-alanine issue and longer than that in a broader context. I think we had demonstrated we had been plenty patient.”

Dan Fabricant, the president and CEO of the Natural Products Association, met with FDA officials in December 2019 to discuss new dietary ingredients—including beta-alanine—being imported into the U.S. whose evidence of safety hasn’t been reviewed by FDA. Fabricant said he was joined at the meeting by Kevin Bell, outside counsel to NPA, as well as a representative of Lonza.
Fabricant followed up again with Yiannas on Jan. 8, and though the holiday season had recently ended, he said he “didn’t want to lose any momentum on our [December 2019] meeting and addressing the import issue of adulterated NDIs.”
“Does it make sense to have a follow-up meeting or phone call in early/mid-February on the matter?” Fabricant asked by email. “In the interim, if there’s anything needed from us, please let us know what we can do on our end.”
The next day, Tave advised Fabricant he was responding on Yiannas’ behalf. “At this point, we don’t think it makes sense to schedule a follow-up meeting or phone call yet,” he wrote in an email. “We will not hesitate to reach out to you if we have any questions or need any additional information. We very much appreciate your collaboration on this important issue.”
On Feb. 24, Bell sent Tave an eight-page letter, requesting FDA enforce against “adulterated” beta-alanine being imported into the U.S. The letter also included two attachments: FDA’s Feb. 1, 2019 letter to Bell, acknowledging NAI’s NDIN for CarnoSyn beta-alanine; and a list of 24 Chinese manufacturers and exporters of beta-alanine, which identified their addresses, websites, kilograms of beta-alanine exported to the U.S. and total shipments between Feb. 1, 2019 and Jan. 31, 2020.
“Despite being a responsible stakeholder in the dietary supplement industry for over 40 years, since receiving its AKL [acknowledgement without objection] letter from FDA, NAI continues to be negatively impacted by scofflaws exporting adulterated, generic forms of beta-alanine to the U.S. and FDA’s lack of enforcement of NDIN requirements,” Bell wrote to Tave.
Fabricant followed up again in May with Yiannas concerning “adulterated imported ingredients,” requesting a teleconference to discuss "next steps." He also expressed interest in exploring what FDA actions would result from the May 2019 public meeting.
Nina Zimdahl, special assistant to Yiannas, responded Yiannas was “primarily focused on COVID-19” and could not meet at the time. She said FDA would “be in touch when the public health emergency subsides if we have any questions for you on this issue or we have more to share with you on NDIs and imports.”
In a June 8 email to FDA officials, Fabricant forwarded them an email sent to LeDoux about a company in China selling beta-alanine.
“It's clear from the thread that this company is currently selling their adulterated ingredient made with ‘advanced enzyme catalysis, metabolic engineering and biological fermentation technology,’” Fabricant wrote, suggesting such “chemical changes” would require an NDIN based on draft guidance published by FDA in 2016.
Tave thanked him for sharing the information with his agency. Fabricant confirmed this was his most recent correspondence with FDA regarding beta-alanine.
‘Just a ruse’
Bell is disappointed FDA has not taken enforcement action against beta-alanine, despite all the conversations and records shared with the agency.
“We were made to believe that Director Tave and other senior FDA officials wanted to move forward on several forms of NDI enforcement,” the lawyer said in an email. “That apparently was just a ruse.”
Tave, FDA’s supplements chief, expressed a considerably different perspective. While he acknowledged NAI and its advocates provided many records to FDA, he maintained the information shared with the agency was not tied to violations of law.
“We met with them multiple times whenever they asked to meet,” he said in an interview. “We reviewed what they sent whenever they asked us to review something, but we don’t have the capacity to do their job.”
About the Author
You May Also Like

