Safeguard your supply chain and set yourself apart in a competitive global market by verifying your ingredients.
September 29, 2019

Sponsored Content
During a climate of increased government scrutiny and consumer concerns about the quality of drug and dietary supplement ingredients, USP’s Verification Program helps manufacturers and suppliers protect public health through rigorous testing, review of quality control documentation and auditing processes, USP evaluates excipient, pharmaceutical and dietary ingredients for identity, purity, potency, performance characteristics, and quality.1
An Accelerating Pace of Change
With society’s increasing incidence of lifestyle diseases, rising healthcare costs, and an emphasis on paying for value, we’re seeing a paradigm shift from curative care toward preventive health management practices and personalized approaches to healthcare.
Keeping Up with Market Growth
The pace of medical advancement is as exciting as it is dizzying. The market for affordable medicine is expanding as expiring patents of major drugs are paving the path for more generic products. With these fast-moving medical innovations come hopes for better outcomes and quality of life, but also their own unique set of manufacturing and regulatory challenges.
As with every industry, there are good and bad actors. There will be suppliers and manufacturers who take shortcuts and compromise the quality of their ingredients and products. On the flip side, there will be companies committed to making quality products for consumers. Now there is a clear way for suppliers and manufacturers to receive guidance on safeguarding their supply chain, assuring the quality of their products and gaining competitive position and brand recognition for their efforts. It starts with the USP Verification Program.
Start with Standards to Ensure Quality is Built into Your Products
Since 1820, USP has helped improve the quality of drugs and their ingredients. Launched in 2001, the USP Verification Program has offered third-party quality evaluation to help manufacturers improve quality across:
Dietary Ingredients
Excipients
Active Pharmaceutical Ingredients
Offered to manufacturers and brands worldwide, USP Verification Services annually evaluates the quality of verified products through the three-step process:
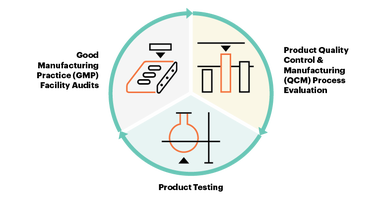
The USP Verification Services’ multi-step process provides increased confidence in supplier qualification, multiple snapshots of the quality management system, and product testing that helps manufacturers maintain an edge in an increasingly competitive global market.
The USP Verification Program provides an independent third-party verification of the quality of a supplier’s ingredient. Verification goes beyond a paper or on-site GMP audit to evaluate not only what the supplier is doing to control quality, and how those controls are implemented, but why those are the correct controls to ensure product quality.
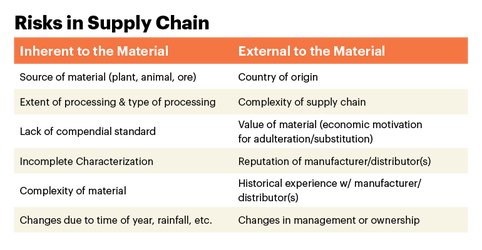
Have You Safeguarded Your Supply Chain?
A robust risk assessment of raw material quality and its supply chain evaluates internal and external risks and provides tailored risk mitigation strategies.
The Benefits Are Clear
With risks to the supply chain presenting themselves from multiple sources, it only makes sense to elicit feedback from multiple sources to mitigate and manage them. The most trusted verification programs are those offered by independent third-party organizations that avoid bias. USP Verification Programs deliver on all these fronts.
By participating in the USP Verification Program, you mitigate risks, confirm ingredient quality, and give confidence to buyers who know they can trust the quality of your products. SP Verified
Demonstrate compliance with applicable Good Manufacturing Practice (GMP) requirements with manufacturing facility audits
Verify conformance to appropriate specifications for identity, strength, purity and quality; meet acceptable limits for impurities and contaminants with laboratory testing of ingredient samples
Ensure ingredient consistency from batch to batch with reviews of quality control and manufacturing product documentation
Confirm that USP-verified ingredients continue to meet program requirements with annual risk-based audits and tests of randomly selected lots with ongoing change monitoring and surveillance
USP’s drug standards are FDA-enforceable per the Federal Food, Drug, and Cosmetic Act, and its dietary supplement standards are recognized in the 1994 Dietary Supplement Health and Education Act. As an independent entity, USP is able to work with governments, manufacturers, and practitioners worldwide to set public health standards that are trusted worldwide.
Set yourself apart. Discover more about our Verification Program at usp.org/ivp
1 USP has tested and verified select dietary supplements for their ingredients, potency and manufacturing process. USP does not verify efficacy claims. See www.USPverified.org
You May Also Like